UDI: Unique Device Identification
Identify, Track, and Manage Medical Devices Throughout their Lifecycle
The FYI on UDI
UDI, or Unique Device Identification, is a system developed and mandated by the FDA to accurately find, track, store, and manage medical devices throughout their distribution and use. Every device that is produced and sold in the healthcare market must be labeled with a different numeric or alphanumeric code that contains specific information about its manufacturer, expiration date, and more.
Like many barcoding systems, each identifier will have two components- machine-readable, for instant computer-based recognition, and human-readable, to serve as a “two point check” that the device is valid.
UDI compliance can be intimating if you aren’t sure where to begin. Paragon has the expertise to assist you in designing and implementing your compliant system. Give us a call at 800.211.0768 or contact us here to have an expert assess your current set up.
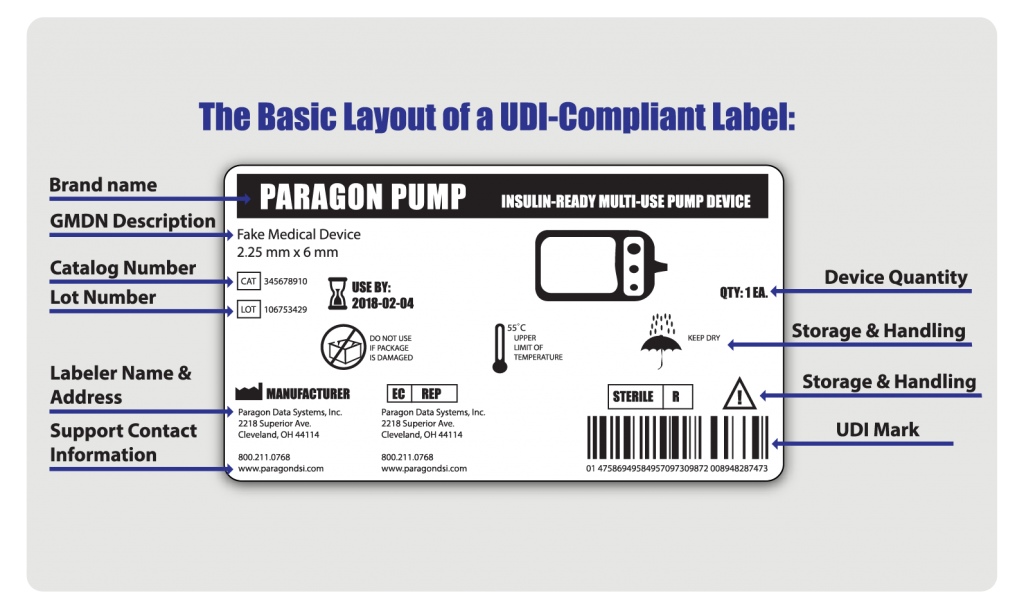
To be in compliance, every UDI label must have the following:
-
-
- A device identifier (DI): a fixed label that identifies the manufacturer and the exact version or model of a device
- A production identifier (PI): a changeable label that identifies one or more of the following:
- lot or batch number
- serial number
- expiration date
- date of manufacture
- the distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P)
-
What Medical Devices Require Unique Device Identification?
A medical device refers to instruments or apparatuses that are implanted within the body or used to diagnose, prevent, or treat disease or other conditions, and do not carry out processes through chemical action.
Class I Devices:
- Not meant to support or sustain life
- Are not considered substantially important to preventing healing or promoting good health
- May not present an unreasonable risk of illness or injury
Examples of Class I Devices include surgical gloves, surgical instruments, and bandages.
Class II Devices:
- General safeguards cannot assure complete safety or treatment effectiveness
- Used in tandem with other methods or devices to assure effectiveness
- Pose a minimal risk to health and safety
Examples of Class II Devices include infusion pumps and powered wheelchairs.
Class III Devices:
- Meant to support or sustain life
- Considered substantially important to promote healing and good health
- Present a potential, unreasonable risk of illness or injury
Examples of Class III Devices include implantable pace makers, external defibrillators, and HIV testing equipment.
Who Needs to Comply with this Mandate?
Almost all medical device manufacturers need to be aware of these regulations and remain compliant with the mandate. However, the FDA also requires that anyone identifying as a “labeler” must also comply. You are a labeler if you apply a label to a device or ask for the label to be modified, and intend to commercially distribute the product without further replacing or altering the label. Most often, this “labeler” would still be the device manufacturer, but in other cases the labeler is defined by other criteria, such as a contract sterilizer or specification developer.
Simply put, if your company produces or distributes medical devices for commercial use anywhere in the United States, you are required to register your business every year with the FDA.
How Do I Obtain My UDI Code?
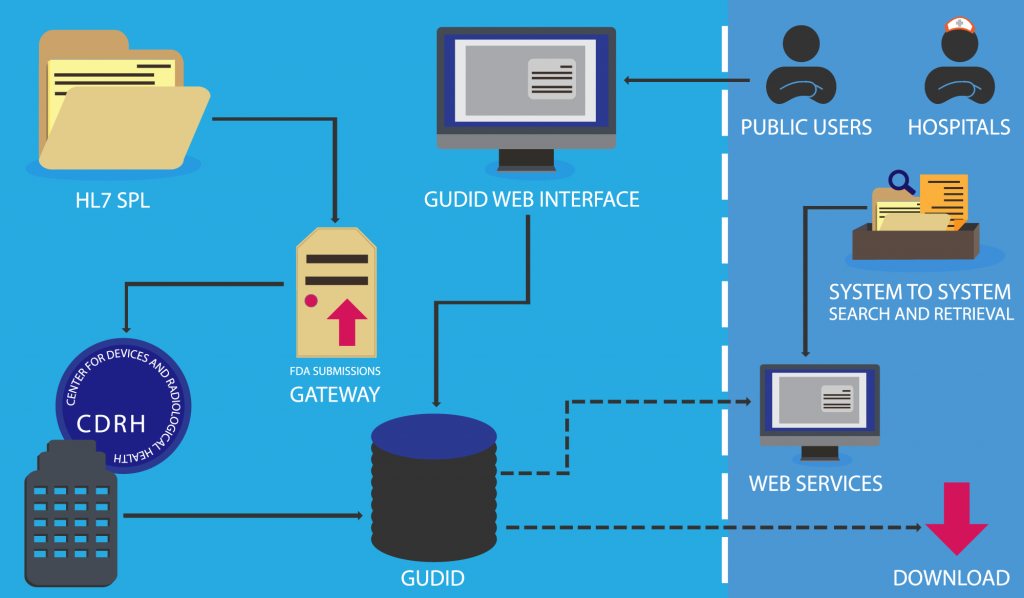
As the manufacturer, you do not create the device identifier (DI). Manufacturers are asked to submit information about their device to the Global Unique Device Identification Database (GUDID), an organization working under the FDA, who will assign the code for you.
Submission Options:
There are two ways to submit your device information to the GUDID: the GUDID Web Interface, and HL7 SPL xml File Submission. Submission of a device requires that a company establishes a GUDID account, regardless of the option chosen.
The information included in a submission packet is as follows:
- Company’s DUNS number
- Company’s name
- Regulatory contact information
- Name
- Phone
- Address
- Labeler DUNS for the GUDID Account
- Coordinator Information
- Any necessary third-pary DUNS numbers
At minimum, every GUDID account must have:
- One regulatory contact
- One Labeler DUNS number
- One Coordinator
The submission modules have been outlined below:
After UDI Submission
Public Access to your Unique Device Identification Information
Once device information has been submitted to the FDA, it is retained in the Global Unique Device Identification Database (GUDID). The device identifier (DI) is the “primary key” and is used to look up product information in the GUDID. The entirety of a device’s production identifiers (PI) are not stored in this database; rather, the GUDID only contains PI flags to explain basic device attributes.
Once your device has been submitted, approved, and recorded, you and your device will be listed within the database. Some of this information is contained in a separate database called AccessGUDID, which is open to hospitals, patients, and the general public.
Why UDI?
While UDI compliance is mandatory, there are still many other benefits to using this system besides avoiding fines from the government. Unique Device Identification will increase efficient data capture and record-keeping processes and improve the value you can offer your own customers.
Data Accuracy and Precision
Because UDI labels allow for more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
Improved Health and Safety
UDI markings reduce diagnostic and treatment errors by allowing health care professionals to quickly and accurately identify a device, the device’s purpose, and the device’s risk factors to the patient.
Standard Identification
This mandate standardizes medical documentation to globally recognized criterion across many different platforms of use (such as EMRs, claim data sources, and device registries), which provides a more secure way to distribute authentic products and prevent device counterfeiting.
Efficiently Manage Recalls
A UDI gives manufacturers, distributors, and medical providers a way to implement a recall quickly, reducing the amount of harm a design flaw can cause.